Miljöpåverkan
Sevofluran
Miljörisk:
Användning av sevofluran har bedömts medföra försumbar risk för miljöpåverkan.
Nedbrytning:
Sevofluran är potentiellt persistent.
Bioackumulering:
Sevofluran har låg potential att bioackumuleras.
Läs mer
Detaljerad miljöinformation
Detaljerad miljöinformation / Detailed background information
Due to the application of the medicine and its physical-chemical properties, the parent compound sevoflurane (CAS 28523-86-6) is mainly emitted to the air compartment. Indeed, a minor amount of sevoflurane and its metabolites are maybe emitted down-the-drain. Only the latter releases, parent and metabolite, have been examined in the following risk classification. Thus, only the potential risk to the aquatic compartment is addressed here.
Environmental Risk Classification
Predicted Environmental Concentration (PEC): parent compound
PEC is calculated according to the following formula:
PEC (μg/L) = (A*109*(100-R))/(365*P*V*D*100) = 1.5*10-6*A(100-R)
PEC = 0.000112 μg/L
Where:
A = 7359.4 kg * 0.01% (total sold amount API in Sweden year 2018, data from IQVIA (2019); reduced by fraction excreted). The 0.01% represents a worst-case assumption; Justification on reduction see metabolism data below.
R = removal rate (due to loss by adsorption to sludge particles, by volatilization, hydrolysis or biodegradation) = 0 %. This value represents a worst-case assumption due to volatilisation; Justification see data below.
P = number of inhabitants in Sweden = 9 *106
V (L/day) = volume of wastewater per capita and day = 200 (ECHA default) (ECHA, 2016)
D = factor for dilution of waste water by surface water flow = 10 (ECHA default) (ECHA, 2016)
Predicted No Effect Concentration (PNEC): parent compound
Ecotoxicological studies
For sevoflurane information on acute aquatic toxicity on Algae, Fish and Daphnia magna, 3 studies according to OECD 201, 202 and 203 (Ref: Claude MB & Krueger HO, 2008a, Claude & Krueger HO, 2008b, Claude & Krueger HO, 2008c) are given by AbbVie on the Fass website. However, as the primary study reports are not available, the reliability of the studies result could not be reviewed.
Algae (Pseudokirchneriella subcapitata) (OECD 201; Claude & Krueger, 2008a):
EC50 72 h (growth inhibition) > 100 mg/L
Crustacean (Daphnia magna):
Acute toxicity
EC50 48 h (immobilisation) = 48 mg/L (OECD 202 ; Claude & Krueger, 2008b)
Chronic toxicity
Not available
Fish fathead minnow (Pimephales promelas):
Acute toxicity
LC50 96 h (mortality) = 43 mg/L (OECD 203; Claude & Krueger, 2008c)
Chronic toxicity
Not available
AbbVie derived following PNEC:
PNECsurface water = lowest EC50/1000 = 43 µg/L
where 1000 is the assessment factor used. The EC50 for the fathead minnow (Pimephales promelas) has been used for this calculation since it is the most sensitive of the three tested species (algae, crustacean, fish).
This approach is according to REACh Technical Guidance Document, Chapter R.10 (ECHA, 2008) where an assessment factor of 1000 is applied on the lowest acute EC50/LC50 value of three trophic levels.
Environmental risk classification (PEC/PNEC ratio): parent compound
According to the European Medicines Agency guideline on environmental risk assessment of medicinal products (EMA/CHMP/SWP/4447/00), use of sevoflurane is unlikely to represent a risk for the environment, because the predicted environmental concentration (PEC) is below the action limit 0.01 μg/L.
In addition, based on the available information,
PEC/PNEC = 0.000112/43 = 0.0000026, i.e. PEC/PNEC ≤ 0.1 which justifies the phrase «Use of sevoflurane has been considered to result in insignificant environmental risk.»
Predicted Environmental Concentration (PEC): metabolite HFIP
PEC is calculated according to the following formula:
PEC (μg/L) = (A*109*(100-R))/(365*P*V*D*100) = 1.5*10-6*A(100-R)
PEC = 0.0471 μg/L
Where:
A = 7359.4 kg * 168.05 g/mol / 200.05 g/mol * 5% (total sold amount API in Sweden year 2017, data from IQVIA (2018); reduced by fraction excreted). The 5% represents a worst-case assumption; Justification on reduction sees metabolism data below.
R = removal rate (due to loss by adsorption to sludge particles, by volatilization, hydrolysis or biodegradation) = 0 %
P = number of inhabitants in Sweden = 9 *106
V (L/day) = volume of wastewater per capita and day = 200 (ECHA default) (ECHA, 2016)
D = factor for dilution of waste water by surface water flow = 10 (ECHA default) (ECHA, 2016)
Predicted No Effect Concentration (PNEC): metabolite HFIP
Ecotoxicological studies
In the bioaccumulation study (METI, 1985), an acute toxicity to the rice fish (Oryzias latipes) was given. However, detailed information is missing. In addition, results are available in the public literature on the acute toxicity to the fish fathead minnow (Pimephales promelas) (Geiger et al., 1986). A registration dossier under REACh is available on the ECHA website (ECHA, 2018) describing recently performed aquatic toxicity studies with HFIP. It should be noted that the original study reports could not be evaluated. Instead, only information publicly disseminated on the ECHA website served as basis of this assessment. Therefore, definitive quality and reliability cannot be assessed from this limited information. However, the registrants regarded the studies as reliable.
Algae:
ECOSAR predicts an EC50 48h of 126 mg/l for green algae (HFIP is within the applicability domain of the model)
Green alga (Pseudokirchneriella subcapita (synonym Raphidocelis subcapitata) formerly known as Selenastrum capricornutum) (OECD 201, GLP, including analytical monitoring) (ECHA, 2018)
EC50 72 h (growth rate) > 100 mg/L (nominal); > 97.2 mg/L (measured)
NOEC 72 h (growth rate) > 100 mg/L(nominal); > 100 mg/L (measured)
Crustacean:
Acute toxicity
ECOSAR predicts an LC50 48h of 390 mg/l for daphnia (HFIP is within the applicability domain of the model)
Daphnia magna (OECD 202, GLP, including analytical monitoring, limit test) (ECHA, 2018)
EC50 48 h (immobility) > 100mg/L (nominal); > 97.3 mg/L (measured)
Chronic toxicity
Not available
Fish:
Acute toxicity
Fathead minnow (Pimephales promelas)
LC50 96 h (mortality) = 244 mg/L
EC50 96 h (behavioral changes, increased respiration and changes in fish colour) = 177 mg/L; test results based on measured test substance concentrations; flow-through system; initial 117-656 mg HFIP/L nominal (95.9 - 533 mg/L effective); 24.9°C; 5.8 mg DO/l; pH 7.68 (Geiger et al., 1986)
Rice fish (Oryzias latipes) LC50 48 h = 270 mg/L; japanese guideline JIS K 0102; (METI, 1985)
Chronic toxicity
Not available
According to REACh Technical Guidance Document, Chapter R.10 (ECHA, 2008) an assessment factor of 1000 is applied on the lowest EC50/LC50 value of three trophic levels. Experimentally derived data on acute toxicity to fish is available. EC50 for fathead minnow is the more sensitive of the two tested fish species. For daphnia has been performed with 100 mg/L (nominal) and no effects were observed. For algae up to the highest tested concentration (100 mg/L (nominal)) no effects were observed. The 100 mg/L has been used for PNEC derivation as a worst-case estimate.
PNECsurface water = lowest EC50/1000 = 100 µg/L
Environmental risk classification (PEC/PNEC ratio): metabolite
As only experimentally derived acute toxicity data of HFIP to fish is available, risk of environmental impact of sevoflurane cannot be completely excluded, since there is not sufficient ecotoxicity data available.
However, based on the available information,
PEC/PNEC = 0.0471/100 = 0.00047, i.e. PEC/PNEC ≤ 0.1 which justifies the phrase «Use of sevoflurane has been considered to result in insignificant risk.»
Degradation
Biotic degradation
Ready degradability:
For sevoflurane a study according to OECD 301D is given by AbbVie (Ref: Matthews & Schaefer. 2008) on the fass website. However, as the primary study report is not available, the reliability of the study result could not be reviewed.
Test results in ≤ 4.4% degradation in 28 days (OECD 301D; Matthews & Schaefer, 2008).
For the metabolite HFIP biodegradation study was conducted by the Japanese Government for the existing chemicals survey in 1986:
Test results in 0% degradation in 28 days based on the TOC, and 5% degradation in 28 days based on direct quantification (GC) (similar to OECD 301C MITI (I), but with DOC/GC analysis; 30 mg/L standard activated sludge from 10 different places in Japan (no pre-exposure), 25 +/-1 °C, test substance concentration 100 mg/L, solution adjusted to pH 7.0, test solution volume 300 ml, number of replicates n=2, degradation rate of aniline as reference after 7 days 73% based on TOC) (METI, 1986).
These results indicate that sevoflurane as well as HFIP can be regarded as not readily biodegradable.
Inherent degradability:
Information on inherent biodegradability is not available.
Simulation studies:
STP simulation studies and test results in water, sediment and total system are not available.
Abiotic degradation
Hydrolysis:
Experimentally derived information on hydrolysis is not available.
Photolysis:
Experimentally derived information on photolysis in water is not available.
Justification if R is not equal to 0, e.g. modelling results using SimpleTreat:
As sevoflurane is not readily biodegradable, and simulation studies are not available, the default value was used for removal rate R = 0. However, this is a worst-case approach for sevoflurane, as modelling results using SimpleTreat suggest that within STP about 89.3% is emitted to air, 1.88% to sludge, and only 8.82% to water.
As the metabolite HFIP is not readily biodegradable, and simulation studies are not available, the default value was used for removal rate R = 0. Modelling results using SimpleTreat suggest that within STP about 3.8% is emitted to air, 0.939% to sludge, and 95.3% to water.
Justification of chosen degradation phrase:
As sevoflurane as well as its metabolite HFIP are not readily biodegradable, and simulation studies are not available, the medicine is potentially persistent.
Photodegradation:
In the atmosphere Sevoflurane could be removed by chemical reaction with radicals, by photolysis and by wet or dry deposition. The degradation time is assumed to be limited by the reaction with the hydroxyl radical (OH•). The following rate coefficient kOH are described:
Brown et al. (1989): 7.3E-14 cm3/molecule-sec at 300 K
EpiSuite (estimation, AOPWIN): 6.78E-14 cm3/molecule-sec at 298 K
Langbein et al. (1999): 2.7E-14 cm3/molecule-sec at 298 K
Sulbaek Andersen et al. (2010): 1.79E-14 cm3/molecule-sec at 272 K
Sulbaek Andersen et al. (2012): 3.9E-14 cm3/molecule-sec at 298 K
The life-times given in the literature are between 0.6 and 4 years depending on the rate constant, OH radical concentration, and temperature.
For HFIP the estimated rate coefficient kOH is 0.1742E-12 cm3/molecule-sec at 298 K corresponding to a half-life of 92 d (EpiSuite, AOPWIN).
Adsorption and desorption to soil
The soil adsorption coefficient (Koc) of sevoflurane was calculated by ACD/Labs to be 544 L/kg. Estimations via EpiSuite show Koc values of 272.4 L/kg (MCI method) and 63.46 L/kg. (Kow method with a logKow of 1.75). Using the logPow and the recommended QSAR of the TGD for non-hydrophobics, results in a Koc of 209 L/kg. Thus, adsorption of sevoflurane to soil and sediment is assumed to be low.
For HFIP the soil adsorption coefficient (Koc) was estimated via EpiSuite. This estimation results in values of 28.04 L/kg (MCI method) and 27.03 L/kg. (Kow method with a logKow of 1.66). Using the logPow and the recommended QSAR of the TGD for non-hydrophobics, results in a Koc of 76.4 L/kg. Thus, adsorption of HFIP to soil and sediment is assumed to be low.
Volatilisation
Distribution of sevoflurane between air and water (Henry’s law constant) was estimated from the ratio of the vapour pressure to the water solubility. The resulted value of 1870 Pa m3/mole at 25 °C resulting in an air-water partitioning coefficient of 0.378 at 12°C indicates a rapid and significant volatilization from water.
For HFIP an experimental Henrys law constant of 4.31 Pa m3/mol at 25 °C was found in the SRC database (EpiSuite; Rochester & Symonds, 1973).
Bioaccumulation
Bioconcentration factor (BCF):
Bioconcentration study for sevoflurane is not available.
Results of a bioconcentration study conducted by the Japanese Government for the existing chemicals survey in 1986 with the carp Cyprinus carpio indicated that HFIP is not expected to pose a significant risk for bioaccumulation (METI, 1986): Test fish: Carp (average weight: 21.3 g, average length: 9.4 cm, average lipid content 4%, 24-hour medication bathing in the teramicin diffused solution for fish farming at the concentration of 0.005 % in stagnant water, acclimatization 25 °C x 14 days), 100 L Volume glass aquarium, flow-through, water flow: 1155 l /day, dissolved oxygen 1st test vessel 5.2-6.7 and 2nd test vessel 5.7-7.1 mg/l; cultivation period: 6 weeks, 25 +/-2 °C; feed twice a day about 2% of fish weight; concentration of the test substance: 1st test vessel 1 mg/l, 2nd test vessel 0.1 mg/l; analytical method: GC-MS, water analysis twice a week, fish analysis 4 times; the degree of concentration by carp of the test substance was 1.1 to 1.4 times in the 1st concentration division and 1.3 to 2.7 times in the 2nd concentration division. The test fish had no anomaly as the result of appearance inspection.
Partitioning coefficient:
An experimental study according to OECD 107 on the logPow of sevoflurane determined a logPow > 2 (Baxter, 2012). With the analytical method performed in this study it was not possible to detect sevoflurane in the water phase, thus no exact value could be determined. However, this experimentally determined lower limit of the logPow is similar to the calculated values of 2.5 at 25°C (ACD/Labs) and 1.75 (EpiSuite/KOWWIN v1.67).
Sevoflurane: estimated Log Dow = 2.5 at pH 7
For HFIP estimated values for logPow of 1.11 (EpiSuite/KOWWIN v1.68) and 1.57 ± 0.53 (ACD/Labs) could be calculated. Experimental value of 1.66 is given in a generally accepted review (Hansch et al., 1995)
HFIP: Log Dow = 1.66 at pH 7
Justification of chosen bioaccumulation phrase:
Since log Dow < 4 at pH 7, sevoflurane has low potential for bioaccumulation. Moreover, toxicokinetic data from humans show that sevoflurane does not remain in human bodies but is released back into the air.
Since BCF < 500, HFIP has low potential for bioaccumulation. This is supported by the partitioning coefficient, due to log Dow < 4 at pH 7.
Excretion (metabolism)
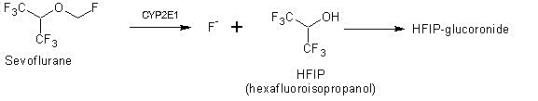
Sevoflurane predominantly leaves the human body via pulmonary exhalation and reaches the atmosphere as the main target compartment. Scientific literature demonstrates that the administered sevoflurane is mainly emitted intact into the atmosphere, and in a lower extent metabolized to fluoride and hexafluoroisopropanol (HFIP), which is rapidly glucuronidated and excreted as HFIP-glucuronide in urine.
Into wastewater, sevoflurane is excreted to < 0.01% as parent compound (Accorsi et al., 2005) and to 2-5% as metabolite hexafluoroisopropanol (HFIP) (Kharasch, 1996) in alignment to SPC Sweden. The pharmacological activity of the metabolite is not known.
A reduction of A (total sold amount API in Sweden 2017) in the PEC calculation is justified based on excretion/metabolism as follows:
Sevoflurane (Parent):
A = 7359,4 kg * 0.01%
HFIP (Metabolite):
A = 7359,4 kg * MW(metabolite)/MW(parent) * M
= 7359,4 kg * 168.05 g/mol / 200.05 g/mol * 5%
PBT/vPvB assessment
As both Sevoflurane and its metabolite HFIP have low potential for bioaccumulation, they do not fulfil the criteria for PBT and/or vBvP substances and thus should not be flagged.
According to the established EU criteria, the medicine should not be regarded as a PBT/vPvB substance.
References
Accorsi A, Morrone B, Domenichini I, Valenti S, Raffi GB and Violante FS, 2005. Urinary sevoflurane and hexafluoro-isopropanol as biomarkers of low-level occupational exposure to sevoflurane. International Archives of Occupational and Environmental Health, 78, 369-378.
Baxter, 2012. Experimental study report: Sevoflurane, Partition Coefficient n-Octanol/Water (OECD 107), Shake Flask Method. Performed by Siemens AG, Prozess-Sicherheit, Industriepark Höchst, B 596 & B 598, 65926 Frankfurt am Main, Germany.
Brown AC, Canosa-Mas CE, Parr AD, Pierce JM and Wayne RP, 1989. Tropospheric lifetimes of halogenated anaesthetics. Nature, 341, 635-637
Claude MB & Krueger HO, 2008a. Sevoflurane: A 72-hour toxicity screening test with the freshwater alga (Pseudokirchneriella subcapitata). Wildlife International, Ltd, Easton, Maryland, project number 161A-108 (cited from information on the fass website by AbbVie; original report not available to Baxter).
Claude MB & Krueger HO, 2008b. Ultane Sevoflurance: A 48-hour static acute toxicity screening test with the Cladoceran (Daphnia magna). Wildlife International, Ltd, Easton, Maryland, project number 161A-109 (cited from information on the fass website by AbbVie; original report not available to Baxter).
Claude MB & Krueger HO, 2008c. Ultane Sevoflurance: A 96-hour static acute toxicity screening test with the fathead minnow (Pimephales promelas). Wildlife International, Ltd, Easton, Maryland, project number 161A-110 (cited from information on the fass website by AbbVie ; original report not available to Baxter).
ECHA, European Chemicals Agency, 2008. Guidance on information requirements and chemical safety assessment. Guidance on information requirements and chemical safety assessment
ECHA, European Chemicals Agency, 2016. Guidance on information requirements and chemical safety assessment chapter R.16: Environmental exposure assessment version 3.0 February 2016. Guidance on information requirements and chemical safety assessment chapter R.16: Environmental exposure assessment version 3.0 February 2016.
ECHA, European Chemicals Agency, 2018. REACH-Registration dossier 1,1,1,3,3,3-hexafluoroisopropanol (CAS 920-66-1), last modified 2018-02-21. European Chemicals Agency, Helsinki, Finland. Publicly available under: REACH-Registration dossier 1,1,1,3,3,3-hexafluoroisopropanol (CAS 920-66-1)
ECOSAR. Calculation Program. U.S. Environmental Protection Agency. OPPT-Risk Assessment Division. Washington, USA.
Geiger DL, Poirier SH, Brooke LT and Call DJ, 1986. Acute toxicities of organic chemicals to Fathead minnows (Pimephales promelas), Vol. III. Center for Lake Superior Environmental Studies, University of Wisconsin-Superior. Supported by U.S. Environmental Protection Agency, 41.
Hansch C, Leo A, Hoekman D, 1995. Exploring QSAR. Hydrophobic, electronic, and steric constants. American Chemical Society, Washington, DC (USA)
IQVIA, 2018. Consumption assessment in kg for input to enviromental classification - updated 2018 (data 2017), Project 1048212
Kharasch ED, 1996. Metabolism and toxicity of the new anesthetic agents. Acta Anaesthesiologica Belgica, 47, 7-14.
Langbein T, Sonntag H, Trapp D, Hoffmann A, Malms W, Roth EP, Mors V and Zellner R, 1999. Volatile anaesthetics and the atmosphere: atmospheric lifetimes and atmospheric effects of halothane, enflurane, isoflurane, desflurane and sevoflurane. British Journal of Anaesthesia, 82, 66-73
Matthews ME & Schaefer EC, 2008. Sevoflurane: Closed Bottle Test, Wildlife International, Ltd, Easton, Maryland, project number 161E-104 (cited from information on the fass website by AbbVie; original report not available to Baxter).
METI, 1985. Test Report - 1,1,1,3,3,3-Hexafluoro-2-propanol (test substance No. K-451)'s degree of concentration test by carp. 26 July 1985, Chemicals Evaluation And Research Institute, Japan (CERI), Chemicals Safety Research Center (results of existing chemicals survey conducted by the Japanese Government)
METI, 1986. Test Report - 1,1,1,3,3,3-Hexafluoro-2-propanol (test substance No. K-451)'s degradation test by microorganisms. 20 February 1986, Chemicals Evaluation And Research Institute, Japan (CERI), Chemicals Safety Research Center (results of existing chemicals survey conducted by the Japanese Government)
Rochester CH & Symonds JR, 1973. Thermodynamic studies of fluoro alcohols. 1. vapor pressures and enthalpies of vaporization. J. Chem. Soc. Faraday Trans. 1, 69, 1267-73.
Sulbaek Andersen MP, Nielsen OJ, Karpichev B, Wallington TJ and Sander SP, 2012. Atmospheric Chemistry of Isoflurane, Desflurane, and Sevoflurane: Kinetics and Mechanisms of Reactions with Chlorine Atoms and OH Radicals and Global Warming Potentials. J Phys Chem A. 116(24):5806-5820
Sulbaek Andersen MP, Sander SP, Nielsen OJ, Wagner DS, Sanford TJ, Jr. and Wallington TJ, 2010. Inhalation anaesthetics and climate change. British Journal of Anaesthesia, 105, 760-766.